Introduction
Thymoglobulin® and Grafalon® are 2 polyclonal rabbit anti-thymocyte globulin (ATG) used in allogeneic hematopoietic stem cell transplantation (allo-HSCT) to prevent graft rejection and graft-versus-host disease (GvHD). Differences in manufacturing lead to different types and concentrations of antibodies in each product. Few studies compare their effects on post-transplant immune reconstitution: indeed, the latter is essential to prevent post-transplant infections as well as relapse (in case of hematological malignancies).
Methods
We conducted a retrospective study on children or adolescents who received a first unrelated allo-HSCT for an Acute Lymphoblastic Leukemia (ALL) between 2007 and 2018, in the department of pediatric hematology of the Robert Debré Hospital in Paris, France. During this time period, 2 types of ATG were used: Thymoglobulin® at 7.5mg/kg or Grafalon® at 60mg/kg. We included patients with a B or T phenotype ALL aged 2 to 18 years at the time of HSCT indication. Stem cell source could be from unrelated matched or mismatched bone marrow, peripheral blood or cord blood. All patients received a myeloablative conditioning regimen consisting of 12 grays total body irradiation and etoposide (60mg/kg).
We compared patients for engraftment, counts of total lymphocytes, B and T lymphocytes, T CD4+ and CD8+ lymphocytes and their sub-types (naïve, effector memory, central memory and effector memory RA+), regulatory T cells, NK cells, and compared lymphocyte proliferation assays at 1, 3, 6 and 12 months after transplant. We used joint models of longitudinal and survival data to further analyze evolution of total lymphocytes, B and T cells, T CD4+ and T CD8+ cells, and NK cells. Finally, we compared clinical outcomes between the 2 groups.
Statistical analyses were performed according to EBMT guidelines (Iacobelli, 2013).
Results
76 children were included: 23 received Thymoglobulin® and 53 received Grafalon®. Stem cell source was peripheral blood or bone marrow for 61 patients and cord blood for 15 patients. Initial characteristics of patients, disease status and HSCT were similar between the two groups except for the median total nucleated cells and CD34+ progenitor cells of the graft, that were significantly higher in the Grafalon® group.
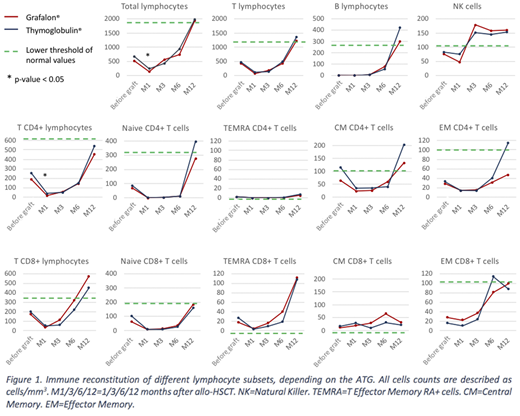
We found a delayed cumulative incidence of engraftment in patients who received Grafalon® compared to those who received Thymoglobulin®: 29 days [interquartile range 22-36] versus 22 days [19.5-29.5], p=0.004. We observed significantly higher counts of total and T CD4+ lymphocytes 1 month after transplant in patients who received Thymoglobulin® as compared to those who received Grafalon®: 238/mm3 [172-466] versus 137/mm3 [51-306] respectively (p=0.005), and 40/mm3 [31.5-70.5] versus 16/mm3 [2.5-41.5], respectively (p=0.011). No differences in immune cell population counts were found 3, 6 and 12 months after transplant (figure 1). However, joint models found no difference in the evolution of lymphocyte subsets analyzed depending on the ATG used, except a non-significant difference in slopes over time for CD8+ cells (p=0.13).
In patients given Thymoglobulin® compared to those given Grafalon®, we found a higher proliferation in response to stimulation by CD3 3 month after HSCT (CD3 index 20.4 [12.4;41] versus 5.9 [3.3;6.4] respectively, p=0.0039), and a higher proliferation in response to stimulation by adenovirus (ADV) antigen 1 year after HSCT (ADV index 78.6 [58.8;163.1] versus 15.7 [2.8;59.3] respectively, p=0.027).
We found no effect of ATG type on overall survival (OS), disease-free survival (DFS), treatment-related mortality and chronic GvHD. In a multivariate analysis, we observed no effect of the type of ATG on OS and DFS.
Conclusions
We found a higher count of total and T CD4+ lymphocytes 1 month after unrelated allo-HSCT for ALL in children who received Thymoglobulin® at 7.5mg/kg compared to those who received Grafalon® at 60mg/kg. From 3 months after HSCT, we found no difference in immune reconstitution. Joint models for longitudinal data and survival found no significant difference in evolution of the lymphocyte subsets analyzed. Clinical outcomes were similar between the two groups. However, we compared an intermediate dose of Thymoglobulin® to a high dose of Grafalon® that is not still the standard in clinical practice. A prospective study comparing Thymoglobulin® to a lower dose of Grafalon® would be needed.
Baruchel:Novartis: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Bellicum: Consultancy. Dalle:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi-Genzyme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bellicum: Consultancy, Honoraria; Medac: Consultancy, Honoraria; Orchard: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; bluebird bio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria; AbbVie Pharmacyclics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal